The Gaseous State
We have a pretty good understanding of the gaseous state in terms of the link between the variables describing the microscopic (atomic-scale) realm, and the variables describing the macroscopic realm.


In the microscopic world we can talk about position, velocity, and mass of the individual atoms, all of which are governed by Newton's laws of motion. By the use of statistical mechanics and averages we can then relate these variables to the macroscopic world by talking about such quantities as pressure, volume, temperature, and moles. For now, though, let's focus on the macroscopic world and search for an equation of state that will tell us the relationships between all these macroscopic world quantities.
Boyle's Law : p V = constant
We start with the experimental observations of Robert Boyle, who showed that the quantities of pressure times volume are a constant for a sample at equilibrium. This is called Boyle's Law. This is shown in the diagrams below.

Each line on the graph is a line of constant temperature, and is called an isotherm. In a plot of versus , we see that the isotherms are straight lines with constant slopes.
For an ideal gas the pressure times the volume is constant, but for real-world gases this is not always true.
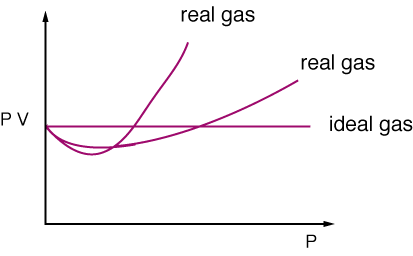
The straight horizontal line in the versus graph is for an ideal gas, while the other two lines are those of some real gases. The thing to notice here is that as pressure goes to zero all gases become ideal, and this is shown by the fact that as goes to zero, all lines converge to the same point. What does zero pressure mean? Zero pressure is a total vacuum. In other words, no gas molecules are present. How can we explain these deviations from Boyle's law? First, let's look at what is happening as the pressure goes to zero. Why do all gases obey Boyle's law at atm? At low pressures the density of gas is low. So, it becomes a rare event for gas molecules to collide. So, a good hypothesis is that Boyle's law only works for molecules that never interact with other molecules.
Okay, then what causes the pV produce to be smaller than the ideal value as the pressure initially increases? This is explained by attractions between gas molecules that alter their trajectories causing them to hit the walls less often. If molecules hit the wall less often, then the pressure will be lower.
And what causes the pV product to be larger than the ideal value as the pressure gets much higher? At high pressures, the density of the gas is higher and collisions between gas molecules become more frequent. This increase in collisions with other molecules cause molecules to hit the wall more often thus leading to a pressure increase. Also, molecules with larger
volumeshave greater chances to collide, so this effect becomes stronger as molecular size increases.
For now, let's go back to ideal gas behavior where is constant. Using these relationships, we can talk about the topic of compressibility and one of the hazards of scuba diving. In the graph below we see that a low compressibility means that something is hard to squeeze, i.e., a big change in pressure gives a small change in volume. Likewise, a high compressibility means that something is easier to squeeze, i.e., a small change in pressure gives a large change in volume.

You can think of compressibility in terms of density. Something that has a high density, like a solid, has a very low compressibility since it takes a lot of pressure to change the volume even slightly, whereas something that has a low density, such as a gas, has a very high compressibility.
Now, let's look at how these ideas relate to diving. It is well known among divers that diving at the surface can be more dangerous than deeper diving. We can understand this by first noting that for every 10 meters you descend in the water, the pressure increases by about 1 atm.

Therefore, when you hold your breath, you create a closed system with your lungs and thus Boyle's law will hold. If you are down at 90 meters (at 10 atm) and you rise 10 meters to 80 meters (at 9 atm), the pressure has decreased by about 10%, and since is a constant your lungs expand by about 10% (probably not too bad). Now if you are at 10 meters (at 2 atm), and you rise 10 meters to the surface (at 1 atm) the pressure had decreased by 50% and expanding your lungs by this factor could cause significant damage, maybe death!
Download
Charles' Law
Now let's look at the relationships between volume and temperature. These relationships were discovered by Jacque Charles and Joseph Louie Gay-Lussac.

In the plot of -vs- above, we observe that the relationship is linear, with an intercept of absolute zero on the Kelvin scale. The slope depends on the pressure and the amount of gas. The dashed lines on the graph are to represent the fact that you can not achieve absolute zero.
Avogadro's Law: V/n = constant
At constant pressure and constant temperature Avogadro's law tells us that .
Emile Clapeyron put Boyle's, Charles', and Avogadro's laws together and came up with the equation of state that we discussed earlier. Thus we know...
- if Temperature is constant, then constant.
- if Pressure is constant, then Volume is proportional to Temperature.
- if Pressure is constant, then Volume is proportional to n (the amount of gas in moles).
Therefore, we can write
is a proportionality constant called the universal gas constant and has the value (Units are important!).
Making the definitions for standard temperature and pressure...
Standard Temperature: K
Standard Pressure: atm
we find using that 1 mole at STP (Standard Temperature and Pressure) has a volume of 22.4 liters.
