Electrochemistry is the branch of chemistry which deals with the chemical changes caused in the matter by passage of electric current and conversion of chemical energy into electrical energy and vice versa.
Electrochemistry deals with the study of electrical properties of solutions of electrolytes and with the interrelation of chemical phenomenon and electrical energies. It is the study of production of electricity from energy released during spontaneous?chemical reactions and the use of electrical energy to bring about non-spontaneous chemical reactions. Chemical changes involving electric current are called electrochemical changes.
Cells and Batteries used by us in various instruments and devices, convert chemical energy into electrical energy. Electrochemical reactions taking place in these cells and batteries are energy efficient and cause less pollution.These electrochemical cells are an important source of energy in today’s time so it becomes very important for us to study electrochemistry for creating new technologies in this field.
Electrochemistry is related to our biological system also. The transmission of sensory signals in our body from brain to other body parts takes place through neurons. This transmission of signals in our body has electrochemical origin. Therefore, electrochemistry is not only limited up to chemistry but its braches extend to physics and biology also. In this chapter we will explore the concepts of electrochemistry in more details under following subtopics:
- Electrolysis and Electrolytic Cell
- Deniell Cell
- Electrochemical Series
- Electrode Potential
- Arrhenius Theory of Electrolytic Dissociation
- Faradays Laws of Electrolysis
- Nernst Equation
- Electrolytic Conductance
- Kohlarusch’s Law
- Concentration Cells
- Applications of Electrolysis
- Commercial Production of Chemicals
- Solved Examples on Electrochemistry
Download
https://drive.google.com/open?id=1o-AWbC1mWNrpy89PpNGrWBG_-MKFYkXRConductors and Non Conductors
Substances around us can be divided into two classes based on their ability of conduct electricity:
- Non-Conductors: Those substances which do not allow electric current to pass through them are called non-conductors or insulators. Example: - wood, plastic glass, rubber etc.
- Conductors: Those substances which allow electric current to flow through tem are called conductors. Examples: Copper, Iron, Gold, Silver, Graphite, salt solution etc.
Conductors can further be divided into two groups
- Metallic Conductors: These conductors conduct electricity or electric current by movement of electrons without undergoing any chemical change during the process. These conduct electricity in both solid as well as molten state. Example: All the metals and Graphite
- Electrolytes: Those substances which conduct electricity only when they are present in aqueous solution and not in solid form are called electrolytes. These conduct electricity by movement of ions in solutions.
Non-ionic compound or covalent compounds do not conduct electricity in aqueous solution and hence they are called non-electrolytes. Examples of non- electrolytes are: Urea, Glucose, Sugar etc.
- Strong Electrolytes are those electrolytes which dissociate completely in aqueous solution to give constituent ions. For example: Inorganic salts like NaCl, KCl, Strong Acid like HCl, H2SO4, Strong bases like NaOH, KOH etc.
- Weak Electrolytes are those electrolytes which partially dissociate in aqueous solution to give constituent ions. For example: weak acid like CH3COOH and Weak bases like NH3.
Comparison of Electrolytic and Metallic Conduction
| S.No | Metallic Conduction | Electrolytic Conduction |
| 1 |
Electric current flows by movement of electrons.
|
Electric current flows by movement of ions.
|
| 2 |
No chemical change occurs.
|
Ions are oxidized or reduced at the electrodes.
|
| 3 |
It does not involve the transfer of any matter.
|
It involves transfer of matter in the form of ions.
|
| 4 |
Ohm's law is followed.
|
Ohm's law is followed.
|
| 5 |
Resistance increases with increase of temperature.
|
Resistance decreases with increase of temperature.
|
| 6 |
Faraday law is not followed.
|
6Faraday law is followed.
|

Pure water does not conduct electricity. Conductivity of tap water is due to dissolved salts and minerals.
Conductivity of metallic conductors increases while that of electrolytes decreases with increase in temperature.
Common salt which we use daily in our food is a strong electrolyte.
Electrolytes are very important in our biological system. Our body requires electrolytes for functioning of nervous system and other life processes.
|
Semiconductors: Semiconductors are those substances whose conductivity is intermediate to those of conductors and insulators i.e. Conductivity is more than insulator and less then conductors. 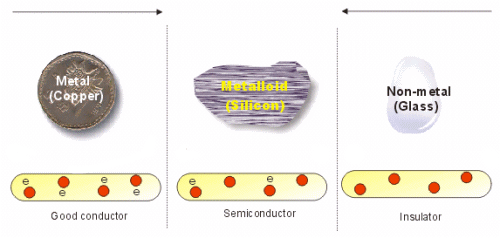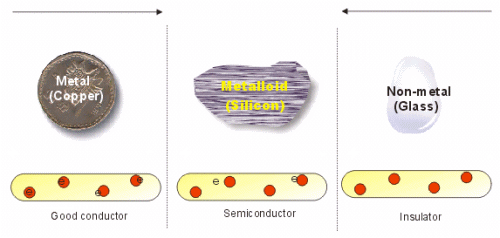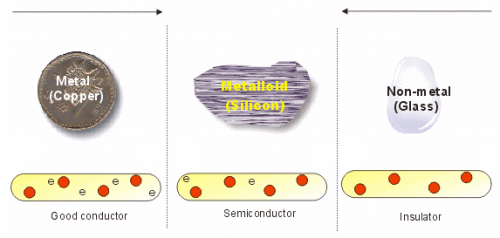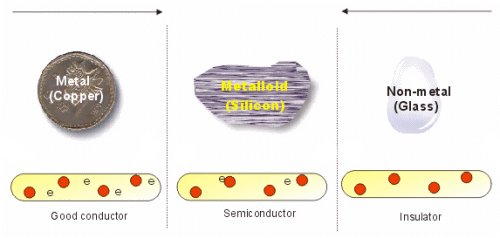

Question 1: Substances which do not conduct electricity in solid state but in question solution is known as..
a. insulators
b. conductors
c. electrolytes
d. strong acids
Question 2: Which of the following substances is not an electrolyte?
a. Common salt
b. Sulphuric Acid
c. Acetic Acid
d. Glucose
Question 3: Which of the following substances is not a non-electrolyte?
a. Gold
b. Mercury
c. Graphite
d.Ammonium Chloride
Question 4: Which of the following compounds will not conduct electricity in its aqueous solution?
a. Carbontetrachloride
b. Silver chloride
c. Sodium acetate
d. Sulphuric Acid
Question 5: Which of the following electrolytes is not strong one?
a. HCl
b. NaCl
c. H2SO4

Q.1
|
Q.2
|
Q.3
|
Q.4
|
Q.5
|
c
|
d
|
d
|
a
|
d
|




